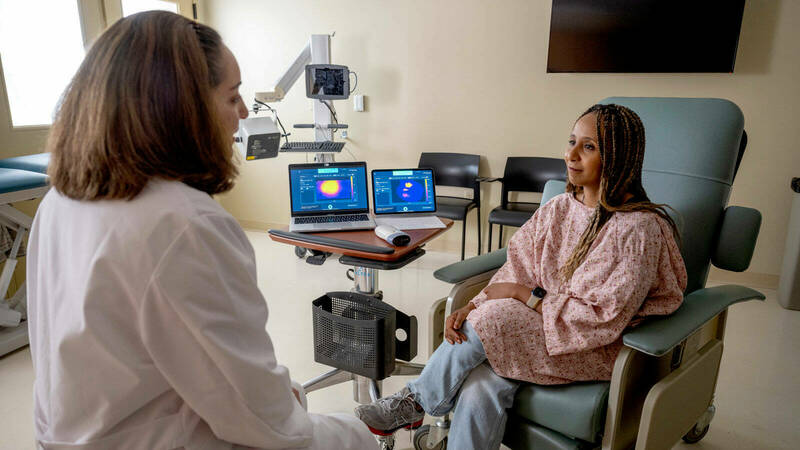
Fighting for breast cancer patients
On March 22, 2011, Jennifer Ehren was sitting in the parking lot of a fabric store, about to buy cloth to make sleeves for her wedding dress. An incoming call halted the errand. The doctor who assessed a small lump in her breast was brief: “We found cancer.”
The weeks after were a blur. “I just became so separate from the world. Life was proceeding for others, but I felt paralyzed with fear and uncertainty,” she recalls. Immediately, she had to consider her future family. Surgery. The wedding. And then, chemotherapy.
“It was brutal,” she says, recalling how allergic reactions to the drugs repeatedly sent her to the hospital in an ambulance. The medical staff was shocked. “I had some of the worst side effects from the chemo the medical staff had ever witnessed.
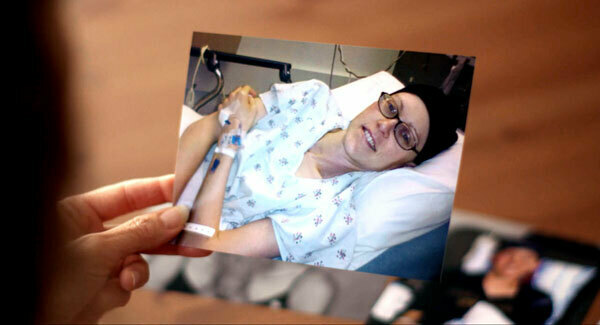
“I didn’t feel like I was fighting cancer,” she says. “I felt like I was fighting to stay alive from chemo.”
At the time, Ehren was a fellow at the Salk Institute studying therapeutics for diabetic complications. Her now-husband, Tom O’Sullivan, was at the Beckman Laser Institute at University of California, Irvine, studying cancer imaging. He went from researcher to patient advocate and it forever changed the trajectory of his work.
During her grueling treatment, what haunted Ehren most was that she didn’t know if the chemotherapy was working, or if enduring the severe reactions would pay off. Statistically, the odds weren’t great. In breast cancer patients receiving neoadjuvant chemotherapy, or treatment before surgery, only about 20 percent see complete disappearance of the tumor, though 60 percent see their tumor shrink. The tumor’s response often dictates the route forward—different medications, surgery, etc.—but testing that tumor comes with a host of other issues and can only be done infrequently.
“A mammogram involves X-ray exposure. MRI requires an injection of a contrast agent. Those are things you can’t do frequently,” explains O’Sullivan, now an associate professor of electrical engineering at Notre Dame. He adds that the costs of these are also prohibitive for patients. O’Sullivan wondered if his work with light and lasers could translate to a safe, easy, and inexpensive diagnostic option.
The result is NearWave: a handheld device that uses light to monitor changes in a tumor.

“The benefit of NearWave scanning is that it can be used frequently without any side effects. We can do this every day, every week. The doctor can do it in their office. You don’t have to go to a special imaging center,” O’Sullivan says, adding that NearWave’s light source has basically no risks. “It’s safer, I’d say, than standing outside in the sun.”
“What we measure with the light are molecular-level changes in the tumor, such as how many molecules of blood, water, fat, and oxygen are present,” O’Sullivan explains. He says that tumors have lower fat and higher water than typical cells, so they can easily detect how the tumor is reacting to treatment almost immediately.
“Less than five days after one chemotherapy injection, we can predict with about 80 to 90 percent certainty whether or not that woman is going to respond to that treatment. So for the ones who are not going to respond, you can spare them the next 12 weeks of chemotherapy and switch them to another drug, or go to surgery, which is going to be much more productive than essentially poisoning them with this chemotherapy.”
It also can help decide the type of surgery—by monitoring the tumor, doctors may be able to opt for a lumpectomy as opposed to a mastectomy if treatment is going well.
The only drawback, O’Sullivan jokes, is that humans aren’t transparent, so light cannot penetrate deeply. Still, the current version of the device can measure tumors up to 3 centimeters deep, with hopes to go further. And while that doesn’t sound like much, O’Sullivan cites data that most breast tumors are within 3 centimeters of the skin surface when the patient is lying flat for assessment, so it should work for most.
Doctor Alice Police, a 30-year breast surgeon, has worked with O’Sullivan since his time at UC Irvine and has seen the project evolve. She led one of the first clinical trials for NearWave’s predecessor and enrolled 70 patients at Northwell Health in New York.
“It was an amazing feeling to me of what a difference it could make in the lives of the patients,” she recalls. Police details that, of the 300,000 people diagnosed with breast cancer each year in the U.S., one-third will undergo chemotherapy.

“[Chemotherapy] is still the hardest part of treatment for patients. It’s way harder than the surgery and the radiation therapy. So what patients want to know is, how do I know it’s working? And the traditional way we can tell if chemotherapy is working is we wait six months after the onset of chemotherapy, and we operate on the patient, and we look at the pathology, and we tell them how well the chemotherapy worked in hindsight through the rearview mirror,” she explains. “And so we’re now going to have a way as early as a week, or even earlier, after the first dose of telling a patient. ‘This is going to work. We have the data to prove it.’ And we can change therapy if it’s not going to work.”
She unabashedly enthuses, “The potential that NearWave has to change things is, I think, extraordinary and unprecedented in the world of breast cancer and chemotherapy.”
***
NearWave is likely one to two years and clinical trials away from hitting doctors’ offices, but its progress is significant. When O’Sullivan joined the faculty at Notre Dame in 2016, he had already participated in clinical trials with places like MD Anderson, University of Pennsylvania, and University of California, San Francisco, and the technology was effective but bulky.
In 2016, shortly after arriving at Notre Dame, he was teaching a biophotonics class on using light in medicine, during which he mentioned his research. After class, a student stayed back and said, “Wouldn’t it be cool if we could take that desktop PC-size thing, and put it in your hand? As easy to use as an ultrasound you can carry around? You could use it potentially in resource-limited settings!”

That student recruited a friend to help work on that idea, which was initially funded by a student grant program through the Harper Cancer Research Institute. That friend, Roy Stillwell ’21 Ph.D., is now the CEO of NearWave. Based in Austin, Texas, the company is already producing and selling the handheld scanners for researchers like O’Sullivan and Police. But NearWave is still partnering with Notre Dame as clinical trials progress with the eventual hope to get FDA approval.
To reduce the size and create NearWave’s sleek, handheld design, O’Sullivan and Stillwell ventured over to the College of Arts and Letters and recruited industrial design professor James Rudolph to draw from his experience designing medical devices and surgical instruments.
Aesthetics aside, the handheld option facilitates easy use in a doctor’s office. Ehren thinks this may eventually allow people to get scanned for breast cancer in their primary care doctor’s office, and get diagnosed, and thus begin intervention, earlier.
Ehren, now in remission, knows firsthand that research is not fast-moving. Still, she reminds her husband of the urgency and utility of NearWave technology. If not for her, if not for thousands of patients worldwide, then for their 7-year-old daughter who will have to be scanned and monitored her entire life due to Ehren’s cancer diagnosis at a young age.
“I want it now,” she says emphatically. “We could help all these people. I mean, come on, let’s get going!”